Clinical trials approved, classified according to the trial area
| Area | No. Clinical Trials |
|---|---|
| Oncology | 575 |
| Hematology | 148 |
| Neurology | 62 |
| Pediatrics oncohemato | 47 |
| Internal medicine 2-Hep. | 42 |
| Internal medicine 1 | 39 |
| General pediatric | 38 |
| Neuroinmunology | 29 |
| Infectious | 24 |
| Nephrology | 23 |
| Pneumology | 19 |
| Cardiology | 17 |
| Intensive care | 14 |
| Endocrinology | 12 |
| Psychiatry | 9 |
| Oftalmology | 7 |
| Pediatrics nephrology | 6 |
| Traumatology surgery | 5 |
| Obstetrics | 5 |
| Anaesthesia | 4 |
| Digestive | 4 |
| Rehabilitation | 3 |
| Urology | 3 |
| General surgery | 3 |
| Others | 2 |
| Dermatology | 2 |
| Gynecology | 2 |
| Plastic / burned | 2 |
| Maxillofacial surgery | 2 |
| Neurosurgery | 2 |
| Neonatology | 2 |
| Genetics | 1 |
| HBP surgery | 1 |
| Immunology | 1 |
| TOTAL | 1,155 |
Clinical trials approved, classified according to the trial area
| Service | No. Clinical Trials |
|---|---|
| ONCOLOGY | 188 |
| VAM | 26 |
| Sicardpath | 19 |
| NEUROSCIENCES | 26 |
| DIGEST. AND HEPA. DISEASES | 20 |
| INFECTIOUS DISEASES | 11 |
| OTHERS | 7 |
| OBST., PEDIAT. AND GENE | 8 |
| SURGERY RESEARCH | 0 |
| TOTAL | 305 |
Other Studies
| EPA | 56 |
|---|---|
| Medical Device (MD) | 7 |
Funding evolution
| 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
| Phase I-II | 4.6M€ | 4,0M€ | 5.8M€ | 6.8M€ | 6.9M€ | 8.6M€ | 9.5M€ | 10.8M€ |
| Phase III | 2.9M€ | 4M€ | 3.6M€ | 4.1M€ | 4.7M€ | 5.9M€ | 6.2M€ | 9.1M€ |
| Phase IV | 0.2M€ | 0.3M€ | 0.9M€ | 0.9M€ | 0.4M€ | 0.4M€ | 0.3M € | 0.2M€ |
| EPA | 0.1M€ | 0.2M€ | 0.3M€ | 0.3M€ | 0.2M€ | 0.26M€ | 0.38M€ | 0.23M€ |
| TOTAL | 7.82M€ | 8.53M€ | 10.51M€ | 12.11M€ | 12.29M€ | 15.15M€ | 16.44M€ | 20.33M€ |
Actives in 2018
| Service | No. Clinicl Trials |
|---|---|
| Industry | 1006 |
| VHIR Researchers | 30 |
| Other Hospitals / Foundations | 119 |
| TOTAL | 1,155 |
Clinical trials approved, classified according to the trial phase
| Phase I-II | 166 |
|---|---|
| Phase III | 128 |
| Phase IV | 12 |
| TOTAL | 305 |
Clinical trials classified according to promoter
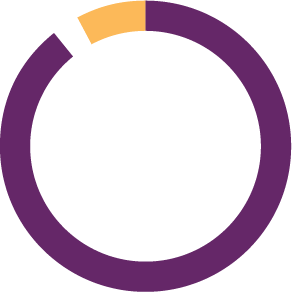
265 | 87% INDUSTRY
5 | 2%VHIR RESEARCHERS
35 | 11%OTHER HOSPITALS / FOUNDATIONS
Clinical trials according to participants
14 | 5% UNICENTER
291 | 95%MULTICENTER




